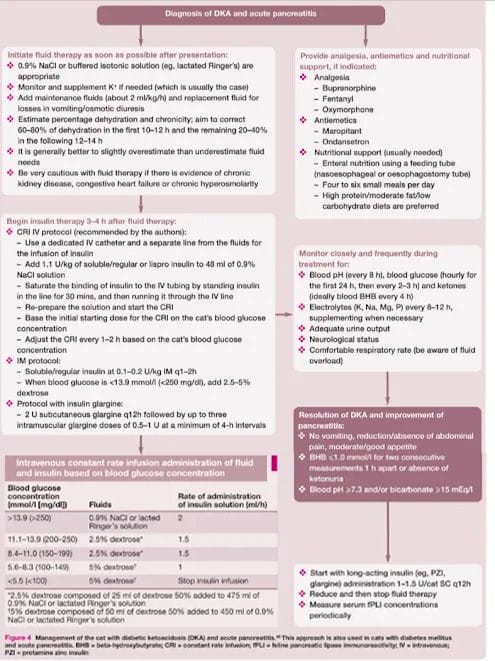
AIM
Prospective block-randomised study was to compare the basal bolus glargine regimen with a regular IV insulin protocol and to demonstrate its utility in the treatment of feline DKA
Case selection
Cats with history of DM (PU/PD with or without polyphagia or wt loss), at least 2 clinical signs consistent with DKA (mentation score >1, vomiting or anorexia ), blood glucose conc above the renal threshold , beta hydroxybutyrate conc >2.55 mmol/L and a metabolic high anion gap (AG) acidosis pH <7.27 and AG >20.6 mmol/L
Exclusion IRIS >3 stage , CHF , mentation score 4 and if clinician did not adhere to the prescribed protocol
Therapy and monitoring
Magnesium was measured in case of refractory hypokalaemia and supplemented with 0.75mmol/kg/24hr
All cats received standard care for DKA including IV 0.9% NaCl, potassium and phosp (if <0.49 mmol/L
Bicarb when <11 mmol/L and mandatory at a pH <6.9 if administered was required to be discontinued at pCo2 (venous) conc >0.51 kPa (38mmHg) to avoid paradoxical cerebral acidosis
For insulin treatment cats were randomly assigned to treatment grps - CRI grp or glargine grp intermittent Sc/Im glargine
CRI grp
units per Kg body weight of regular insulin was added to 250ml bag of 0.9% saline and the first 50ml was drained out to allow for insulin absorption by the plastic tubing. This was iniitated 2 hours after starting rehydration with a CRI of 10ml/h and adjusted every 2 hours as required to achieve a decrease in glucose conc of 2 to 3 mmol/L/h
Glargine
All cats received a bolus of 2 SC units of insulin glargine irrespective of body wight concurrently with starting rehydration and 1 IM unit /cat 2 hours thereafter
IM inj 1 unit per cat were subsequently repeated every 4 hours if glucose was >13.9 mmo/L and Sc glargine was continued every 12 hours with 0.25units/kg based on estimated bwt rounded to the next half or whole unit
The primary aim in both protocols was to achieve a blood glucose conc <13.9 mmol/L. At that point , intensive insulin therapy was continued at 10ml/k but IV F were changed to 2.5-5% glucose containing solution and adjusted to keep BG between 10-13.9 mmol/L
Insulin admin was only decreased to 5ml/hr or stopped in cats with glucose <4.4 mmol/L despite 5% glucose infusions at the calculated infusion rate
In cats with inappetence for more that 3 days a NE tube was placed and the cats were fed 4 times per day
When cats were appropriately hydrated started to eat spontaneously and beta OHB <2.25mmol/L were changed to an exclusively sc insulin regimen ( glargine q12hr with 0.25-0.5 units/kg)
AIM
Compare time to plasma beta OHB conc <2.55 mmol/L between the insulin grps
Time 0 - time IV catheter was placed and infusion therapy was started
Secondary outcome measures were time (h) to improvement of hyperglycaemia and ketonemia a, glucose <13.9, resolution of acidosis pH <7.27, consumption of first meal and discharge from hospital
Measure adverse events - hypoglycemia , severe phyophosphatemia , severe hypokalemia , hyperchloridemia, hypernatremia , severe of moderate hypothermia and bradycardia
Cats were discharged when there was no vomiting and the cars was eating and beta OHB was <2.55 mmol/L and the cats condition was assessed by the clinician das suitable for discharge to the owners
Presence of acidosis did not preclude the cat being discharged if it was caused by concurrent disease eg renal failure
Results
20 cats met all the criteria for inclusion were block randomised to 1 of 2 grps (10 cats per group)
Demographic characteristics including age, sex, weight , body condition score and baseline variables were not significantly different between the grps
17 cats survived to discharge 8 CRI grp and 9 in glargine grp
The time until beta hydroxybutyrate decreased to <2.55 mmol/L was comparable between grps
The glargine-group had significantly shorter median times until first improvement of hyperglycaemia , defined as >1.6 % decrease from baseline and until discharge from hospital
No difference were observed in any other parameter under observation
Non of the cats exhibited Csx of hypoglycaemia
The change of HCT from baseline to discharge was moderately correlated with the lowest phosphorus conc determined during treatment \
The choice of insulin protocol has no influence on changed of HCT , TP ALT , crea , wt
In addition to the first IM insulin injection in the glargine grp a median of 3 (0-11) additional Im glargine injections were administered
Discussion
Findings of this study corroborate the findings of Marshall et al who showed that the basal bolus administration of glargine insulin is a safe and effective alternative to regular insulin CRI protocol currently
As shown by Gallagher et al the application of this basal bolus protocol decreased time of improvement of hyperglycemia and to discharge without affecting survival rate or incidence of adverse events
It has been demonstrated in cats with DKA that despite significant decreases of BG conc >120% within the first 72 hours of conventional therapy , sodium the major determinant of serum tonicity increases and the effective osmolality stats relatively constant minimizing large osmotic shifts
Glargine protocol decreased time to discharge by 1.5 days , shorter time to first meal and quicker resolution of indicators of DKA
Beta hydroxy butyrate measurements are a useful adjunct from monitoring response to therapy and eliminate the need for frequent blood gas analysis which are not specific for ketones
Additionally beta hydroxybutyrate measure facilitate rapid revaluation of diagnosis in cats with low ketone concentrations but ongoing metabolic acidosis
The primary endpoint of treatment in our study was the resolution of ketonemia consistent with the definition of DKA which is a beta hydroxybutyrate conc <2.55 mmol/L
In the absence of comorbidities, conc below this cut off are rarely associated with metabolic acidosis in cats and DKA was not diagnosed at values <3.8 mmol/L
With the resolution of ketonemia to <2.55 mmol/L most of the cats in our study began to eat spontaneously and were transitioned to SC glargine alone
the median time for resolution of ketonemia in our CRI grp was 42 hours compared to 62 and 68 hours in 2 previous studies - possible explanation is the use of a different protocol for adjustment of insulin and glucose administration
In contrast to previous studies insulin infusion was not decreased at glucose conc <16.8 mmo/L or 13.9 mmol/L but insulin admin rate was kept constant until target beta hydroxybutyrate conc were reached
To allow for intensive insulin treatment 5% glucose solution were administered when glucose conc fell below 10mmol/L . This is inline with the latest treatment guidelines in decompensated diabetic children in which a decrease of insulin dose of <0.05 to 0.1 unit/kg/hr is only recommended after DKA has resolved or hypoglycaemia is impending despite the use of 10% or even 12.5% glucose solutions
The early reduction of the CRI insulin dose in the cited studies possibly delayed the resolution of ketonemia and could explain why more key indications were significantly different between the 2 protocols in Gallagher et al
Two retrospective studies compared outcome in cats treated with either 0.05or 0.1 units/kg/h.4,9Whereas the higher insulin dose reduced the odds of poor outcome defined as deaths in the first study,4no differences were found in the second.9Neither of the studies compared time until resolution of ketoacidosis or ketonemia.
A topic of debate in human medicine is whether to routinely start SC administration of a long-acting basal insulin (eg, glargine insulin)at the onset of DKA management. The rationale is to provide stable background insulin concentration and to avoid reoccurrence of hyperglycaemia during the transition time to SC insulin.18,48A common concern raised with SC insulin administration in dehydrated patients is SC insulin accumulation and sudden release after rehydration.49Ameta-analysis of 4 studies in human patients suggests that this concern is likely unwarranted. The addition of SC glargine insulin to standard protocols using IV infusion of regular insulin significantly decreased the time to resolution of DKA, without increasing the risk of adverseevents.50Interestingly, Shankar et al51proposed that the positive effects are caused by yet to be described mechanisms, other than just increased total daily insulin dose. Based on evidence in human and feline patients, some veterinary specialists are combining IV regular insulin infusions with twice- daily SC glargine insulin injections.37Prospective randomized studies are required to more conclusively estimate the benefit of adding SC insulin glargine to DKA protocols for cats
To avoid adverse events associated with hypophosphatemia such as haemolytic anaemia, IV phosphorus supplementation (potassium-phosphate) was a fixed part of our treatment protocol. Severe depletion of phosphorus may lead to ATP depletion in erythrocytes, causing failure of actin and myosin fibres in the cell membranes to maintain normal biconcave structure and deformability.27In our protocol, phosphorus was preventatively administered by providing 25% of potassium as potassium-phosphate. Consequently, hypophosphatemia was observed in only 50% of the cats, compared to 80%,867%,1and 65%4in previous studies, in which phosphorus was administered exclusively to hypophosphatemia patients. Consistent with the expected effects of phosphorus depletion on erythrocyte stability, lower phosphorus concentrations were associated with a greater decrease of HCT from baseline to discharge
Note 4 cats with severe anaemia all had moderate to severe hepatic lipidosis - possible refeeding syndrome which has been associated with hepatic lipidosis and characterised by the developed of severe hypophosphatemia
In conclusion, although the study was underpowered to detect differences in time until resolution of ketonemia, the results suggest that the basal-bolus administration of insulin glargine is a useful alter-native to the current standard CRI-protocol for the management of DKA in cats. It is simple and associated with a shorter time to first improvement of hyperglycaemia and decreased hospitalization time. Additionally,?-OHB measurements using hand-held ketone meters area useful adjunct for monitoring response to therapy and eliminate the need for frequent blood gas analyses. Further studies are required to evaluate the benefits and disadvantages of IM bolus vs CRI proto-cols and to compare choices of fluid therapy in management of feline DKA